While COVID-19 gets most of the headlines these days, another epidemic has been plaguing communities for years before the emergence of the novel coronavirus. The opioid epidemic, which the United States declared a public health emergency in 2017, has impacted millions of lives — many permanently. According to the latest figures from the U.S. Department of Health and Human Services, in one year alone, roughly 10 million people misused prescription opioids and 1.6 million were diagnosed with an opioid use disorder. More than 48,000 people died from an overdose of synthetic opioids other than methadone, with an estimated 14,500 additional deaths attributed to heroin overdose.
An injectable medication, naloxone, is known to rapidly reverse the effects of opioid toxicity if it is administered in time. For people who are alone when an overdose occurs, however, time is not on their side.
A team of University of Washington researchers led by Allen School professor Shyam Gollakota and Dr. Jacob Sunshine, a physician scientist in UW Medicine’s Department of Anesthesiology and Pain Medicine, set out to change that by devising a solution that integrates state-of-the-art computational capabilities with a commercially available, wearable injector platform for subcutaneous drug delivery manufactured by West Pharmaceutical Services. The resulting prototype, which the researchers describe in a paper published today in the journal Scientific Reports, is capable of automatically delivering a life-saving dose of naloxone to a person at the first sign of distress — without waiting for outside intervention.
“The opioid epidemic has become worse during the pandemic and has continued to be a major public health crisis,” said Allen School Ph.D. student and lead author Justin Chan in a UW Medicine news release. “We have created algorithms that run on a wearable injector to detect when the wearer stops breathing and automatically inject naloxone.”
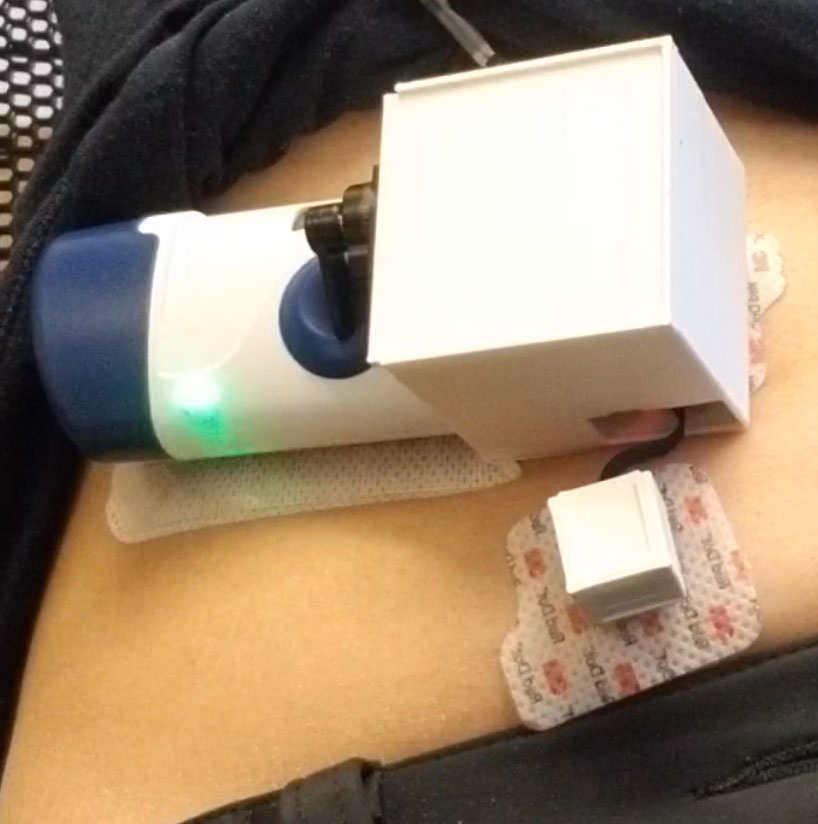
The battery-powered device is designed to be worn on the stomach, similar to an insulin pump, and consists of three parts: the injector system; a sensor patch comprising a pair of on-body accelerometers to detect coarse motion — i.e., movement of body and limbs — and breathing motion, which is attached to a microcontroller running a motion detection algorithm to process the data; and an actuator in the form of a servo motor that automatically activates the injector if the algorithm detects an overdose event. The combination of onboard sensing and lower-power processing enables the device to measure the wearer’s motion as an indicator of their safety in real time — and, most crucially, deliver intervention once that motion has ceased. Built-in Bluetooth capability allows for the option of transmitting data to a companion app loaded onto a nearby smartphone.
“Further work is needed, but this closed-loop system could potentially be transformative if it allowed an unwitnessed overdose to be detected and immediately treated while help was on the way,” noted Sunshine, who is also an adjunct faculty member in the Allen School.
To validate their system, Sunshine, Gollakota, Chan and their co-authors — former UW Electrical & Computer Engineering Ph.D. student and current Allen School professor Vikram Iyer, Allen School alumnus Anran Wang (Ph.D., ‘21), UW Medicine’s Dr. Preetma Kooner, and Alexander Lyness of West Pharmaceutical Services — needed to confirm the accuracy of the sensor measurements and demonstrate that their processing algorithm could distinguish the prolonged apnea events that indicate an overdose to rapidly deliver the naloxone when needed. They accomplished this through carefully constructed studies conducted, with participants’ informed consent, in two different settings: the InSite supervised injection facility in Vancouver, B.C., and the UW Medical Center.
In the first study, at InSite in Vancouver, the team fitted participants with a respiration belt to establish a reference standard for respiratory activity, followed by placement of the sensor patch on the abdomen to collect data on their breathing before, during and after their opioid self-injection, which takes place under medical supervision. During the course of the study, two people experienced apneas post-injection, in the absence of an overdose, which the team’s algorithm correctly identified.
The hospital study involved healthy volunteers who experienced simulated overdose conditions that would trigger the end-to-end system to administer naloxone. Fitted with the prototype injector system, participants were asked to perform a pair of breathing exercises to set a baseline for their respiration, followed by a self-induced simulated apnea event involving holding their breath for 20 seconds. At the 15-second mark, the algorithm successfully registered their simulated overdose condition, leading the actuator to activate delivery of the naloxone. Laboratory analysis of post-delivery blood draws proved that the device had, indeed, delivered naloxone to the individuals as the researchers intended.
This is not the first time Sunshine and Gollakota have teamed up to use technology in response to what some refer to as a silent epidemic. In 2019, the duo and then-Ph.D. student Rajalakshmi Nandakumar, now a faculty member at Cornell University, described a smartphone-based tool that enabled contact-free monitoring of a person’s breathing and movements to detect signs of opioid overdose. The app, which the team dubbed Second Chance, was designed to provide a way for people in danger of overdosing to connect to a friend or first responders. According to Gollakota, the team’s latest work builds on the lessons learned during the app development — while shaving precious minutes off of the potential response time in an emergency.
“With our prior work, we showed how to detect the signs of an overdose. However the big challenge was getting naloxone to the person in a timely manner,” explained Gollakota, who holds the Torode Family Career Development Professorship in the Allen School. “This proof of concept goes a lot further and creates a closed-loop system that can not only automatically detect signs of overdoses but also inject naloxone to reverse the overdose events. This has the potential to be transformative for millions of people who have opioid overdose disorder.”
As a drug delivery device, the auto-injector system would require approval from the U.S. Food and Drug Administration. In consideration of the ongoing public health emergency, the FDA recently released technical guidance for demonstrating the reliability of such emergency-use injector devices. The team also expects additional user studies will be needed to ascertain that people will be able to place and detach such a device without assistance. Gollakota and his colleagues hope that their prototype represents one more step toward making such devices widely available — which could save tens of thousands of lives annually in the U.S. alone.
“We are hopeful it can have a real tangible impact on a real big source of suffering in this country,” Gollakota said.
Read the paper in Scientific Reports here, the UW Medicine news release here, and learn more about the team’s previous work on Second Chance here.




